Spectacular Tips About Is SF2 Polar Or Nonpolar

Understanding Molecular Polarity
1. What is Molecular Polarity, Anyway?
Ever wondered why some things mix well (like sugar in water) and others don't (like oil and water)? The secret often lies in something called molecular polarity. Think of it like magnets — some molecules have a slightly positive end and a slightly negative end, making them "polar," while others are more balanced, making them "nonpolar." SF2, or sulfur difluoride, is one molecule we can explore to understand this concept better. Let's unpack what makes a molecule polar or nonpolar.
Molecular polarity arises from unequal sharing of electrons between atoms in a bond. This unequal sharing happens when atoms have different electronegativities, which is essentially how strongly they attract electrons. A highly electronegative atom pulls the electron cloud closer, creating a partial negative charge (-) on that atom and a partial positive charge (+) on the other. These partial charges are what give a molecule its dipole moment, a measure of its polarity. If these dipoles cancel each other out, the molecule is nonpolar. But if they don't, you've got yourself a polar molecule!
The shape of a molecule is also incredibly important. Even if bonds within the molecule are polar, if the molecule is symmetrical, the individual bond dipoles can cancel each other out, resulting in a nonpolar molecule. Think of carbon dioxide (CO2). Each C=O bond is polar, but because the molecule is linear, the bond dipoles point in opposite directions and cancel. Conversely, water (H2O) has polar O-H bonds, and its bent shape means the dipoles don't cancel, making water a decidedly polar molecule.
So, in a nutshell, molecular polarity is determined by both the polarity of the individual bonds within the molecule and the overall molecular geometry. It's a dance between electron distribution and spatial arrangement that ultimately dictates whether a molecule is polar or nonpolar, influencing its physical and chemical properties. It's like understanding how the notes in a song and their arrangement determine whether the song is harmonious or dissonant. In chemistry, polarity determines how molecules interact with each other and with other substances.

Is SF2 Polar or Nonpolar? The Verdict!
2. Delving into the Polarity of Sulfur Difluoride
Alright, let's get to the heart of the matter: is SF2 polar or nonpolar? To answer this, we need to consider both the polarity of the sulfur-fluorine (S-F) bonds and the shape of the SF2 molecule. Fluorine is one of the most electronegative elements, meaning it really loves to hog electrons. Sulfur, on the other hand, is less electronegative. This difference in electronegativity creates a significant dipole moment in each S-F bond, with fluorine carrying a partial negative charge and sulfur a partial positive charge.
Now, here's where the molecular geometry comes into play. SF2 has a bent or V-shaped geometry. This isn't like carbon dioxide, where the dipoles cancel. Instead, the bent shape means the two S-F bond dipoles don't directly oppose each other. They point in a way that their vector sum results in a net dipole moment for the entire molecule. Think of it like two people pulling on a rope at an angle — the rope will move in the direction of the combined force.
Because the S-F bonds are polar, and the bent molecular geometry prevents the dipoles from canceling, SF2 is a polar molecule! The molecule has a definite positive end (around the sulfur atom) and a negative end (oriented towards the fluorines). This polarity influences how SF2 interacts with other molecules and substances. It's this inherent polarity that gives SF2 its unique chemical characteristics.
In summary, the combination of polar S-F bonds and a bent molecular geometry ensures that SF2 is a polar molecule. This characteristic affects its solubility, boiling point, and how it interacts with other compounds. So, next time you think about SF2, remember its slightly lopsided electron distribution makes it a molecule with character, thanks to its inherent polarity.
Sf2 Polar Or Nonpolar Jujapress
Molecular Geometry
3. How Shape Dictates Molecular Behavior
Molecular geometry, or the shape of a molecule, plays a vital role in determining its overall polarity. Even if a molecule has polar bonds, its shape can cause those bond dipoles to cancel each other out, rendering the molecule nonpolar. Let's explore how different geometries impact polarity and see how SF2 fits into this framework.
Linear molecules, like carbon dioxide (CO2), are straightforward examples. CO2 has two polar C=O bonds, but because the molecule is linear, these dipoles point in opposite directions and cancel, making CO2 nonpolar. Similarly, symmetrical tetrahedral molecules like methane (CH4), despite having polar C-H bonds, are nonpolar because the four bond dipoles cancel due to the symmetrical arrangement of the hydrogen atoms around the carbon. These are examples of how symmetry can neutralize the effect of polar bonds.
However, when a molecule has a bent or trigonal pyramidal geometry, the bond dipoles don't cancel. Water (H2O), with its bent shape, is a classic example of a polar molecule because of this. The two O-H bond dipoles create a net dipole moment, making water a great solvent for polar substances. Ammonia (NH3), with its trigonal pyramidal shape, also exhibits polarity due to the asymmetrical arrangement of its N-H bonds.
SF2, with its bent shape similar to water, falls into the category of molecules where the geometry prevents dipole cancellation. The two S-F bonds are polar, and the bent structure ensures that the dipoles add up to a net dipole moment. Therefore, the molecular geometry of SF2 is a critical factor in determining its overall polarity. So, while bond polarity sets the stage, the shape of the molecule orchestrates the final act, deciding whether the molecule becomes polar or nonpolar.

Real-World Implications of SF2 Polarity
4. Why Polarity Matters in Chemistry and Beyond
Okay, so SF2 is polar. But why does it even matter? Molecular polarity isn't just a theoretical concept; it has significant practical implications in chemistry and other scientific fields. It affects everything from a substance's solubility and boiling point to its reactivity and biological activity.
Polar molecules tend to dissolve well in polar solvents like water, while nonpolar molecules dissolve better in nonpolar solvents like oil. This "like dissolves like" principle is rooted in intermolecular forces. Polar molecules interact strongly with each other through dipole-dipole interactions and hydrogen bonding, which allows them to mix and dissolve easily. The polarity of SF2 means it will be more soluble in polar solvents than in nonpolar ones.
Boiling point is another property influenced by polarity. Polar molecules generally have higher boiling points than nonpolar molecules of similar size and mass. This is because the stronger intermolecular forces between polar molecules require more energy to overcome during boiling. So, we'd expect SF2 to have a relatively higher boiling point compared to similar-sized nonpolar molecules because of its polarity.
Beyond physical properties, polarity also plays a crucial role in chemical reactions. Many reactions involve the interaction between polar molecules or ions. The polarity of a molecule can influence its reactivity and the types of reactions it can participate in. For example, the polar nature of SF2 might make it a good nucleophile or electrophile in certain reactions, depending on the specific conditions. Understanding the polarity of SF2 allows chemists to predict its behavior in various chemical environments and design experiments accordingly.

Comparing SF2 to Other Molecules
5. Polarity in Perspective
To truly grasp the polarity of SF2, it helps to compare it to other similar molecules. Let's consider molecules with similar structures and bonding patterns to see how variations in electronegativity and geometry affect their overall polarity.
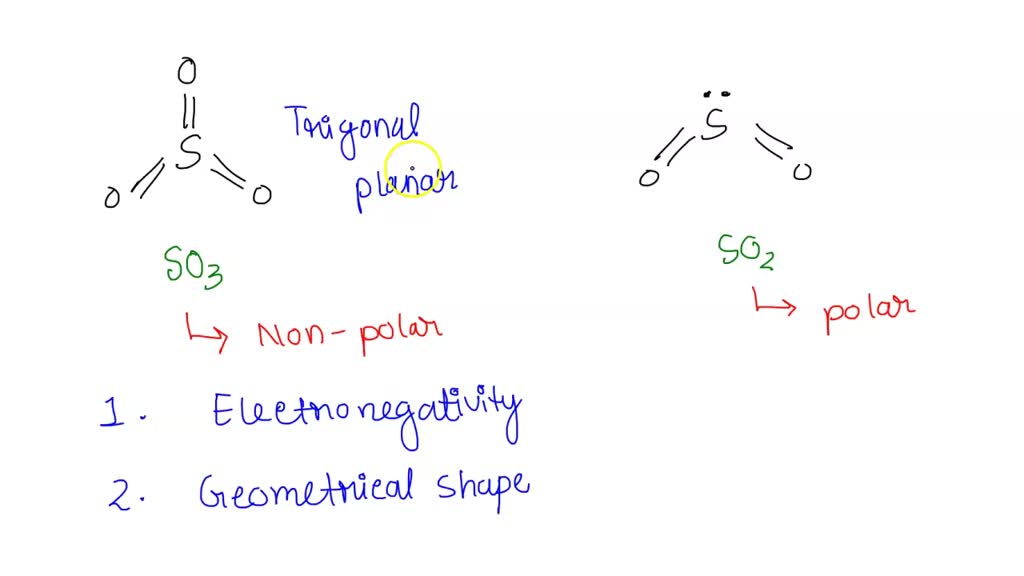
Consider sulfur dioxide (SO2). Like SF2, SO2 has a bent geometry and polar bonds (S-O). Oxygen is also highly electronegative, similar to fluorine. Therefore, SO2 is also a polar molecule. Both SF2 and SO2 demonstrate how bent geometries with polar bonds lead to overall molecular polarity.
Now, let's compare SF2 to carbon disulfide (CS2). CS2 is a linear molecule with two C=S bonds. While the C=S bonds are polar, the linear geometry causes the bond dipoles to cancel each other out, making CS2 nonpolar. This contrasts sharply with SF2, where the bent shape prevents dipole cancellation.
Another comparison can be made with molecules like dichloromethane (CH2Cl2). CH2Cl2 is tetrahedral but the presence of two chlorine atoms and two hydrogen atoms creates an uneven distribution of electron density. The C-Cl bonds are significantly polar and since the molecule isn't perfectly symmetrical, the dipoles do not completely cancel out, rendering dichloromethane polar. This contrasts with methane (CH4), a perfectly symmetrical tetrahedral molecule where the C-H bond dipoles completely cancel out, making it nonpolar. This shows how subtle changes in the molecule structure affect the overall polarity. These comparative examples emphasize how both bond polarity and molecular geometry are crucial in determining whether a molecule is polar or nonpolar, highlighting the specific reasons behind SF2's polar nature.